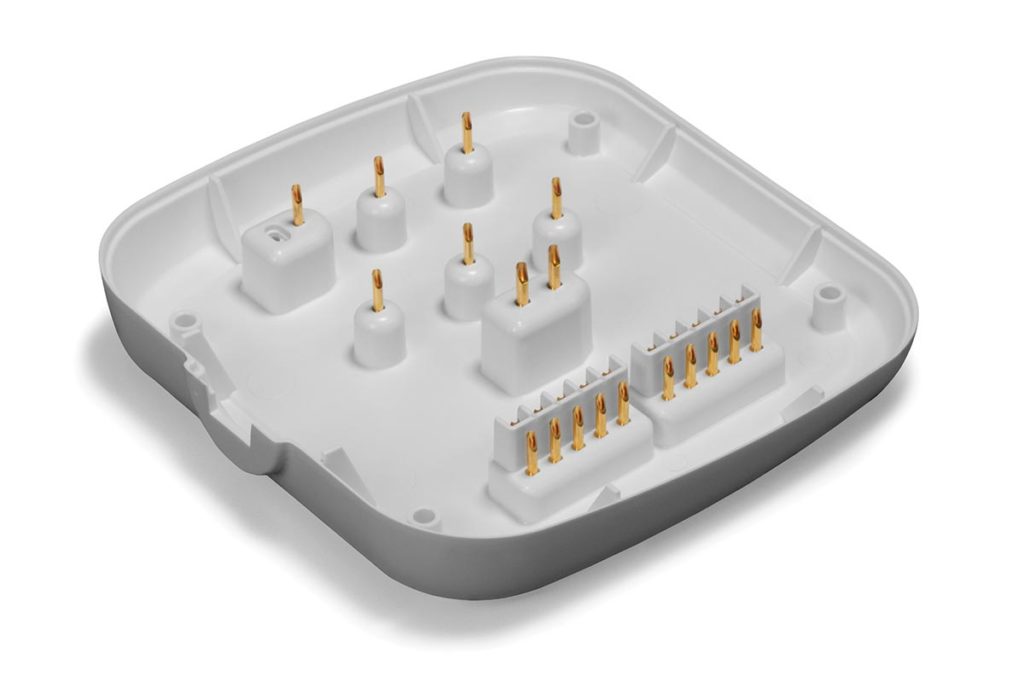
This device is part of a catheter-based system used to treat overactive airway nerves in a procedure called targeting lung denervation. The manufacturing process includes plastic injection molding of polycarbonate to create the front & back housing followed by a multi color secondary pad printing operation.

Trocar device used during a minimally invasive surgical procedure for gas conditioning. Manufacturing process includes plastic injection overmolding an optically clear xylex material on the outer lumen tip, which is a SS tube, along with plastic injection overmolding a Makrolon cap and additional components. Taurus Engineering manages all components for all (4) sizes.

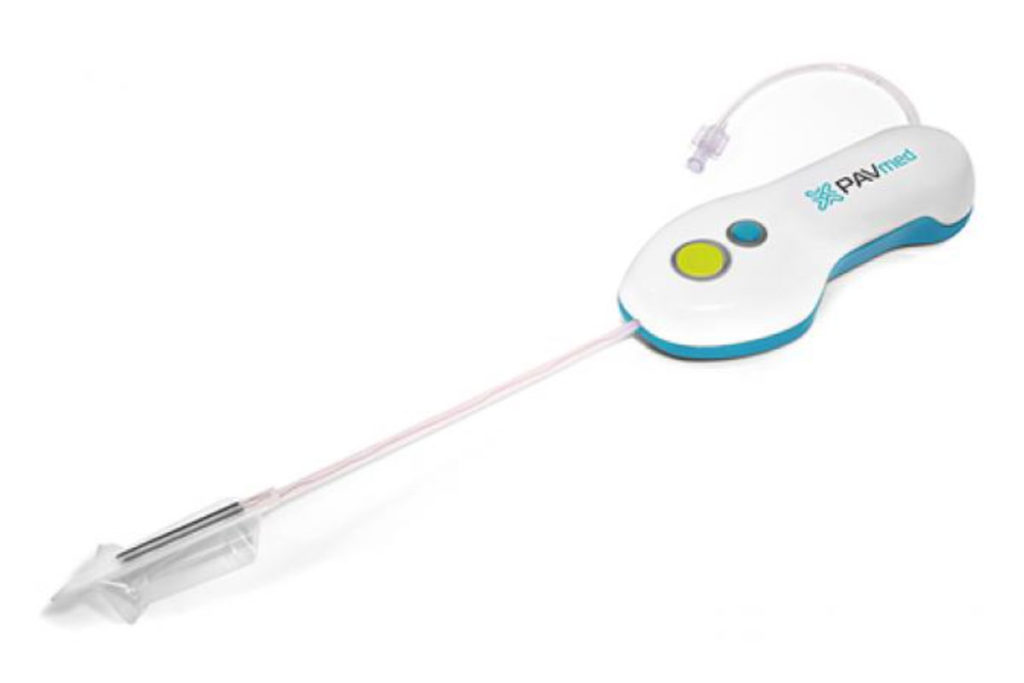
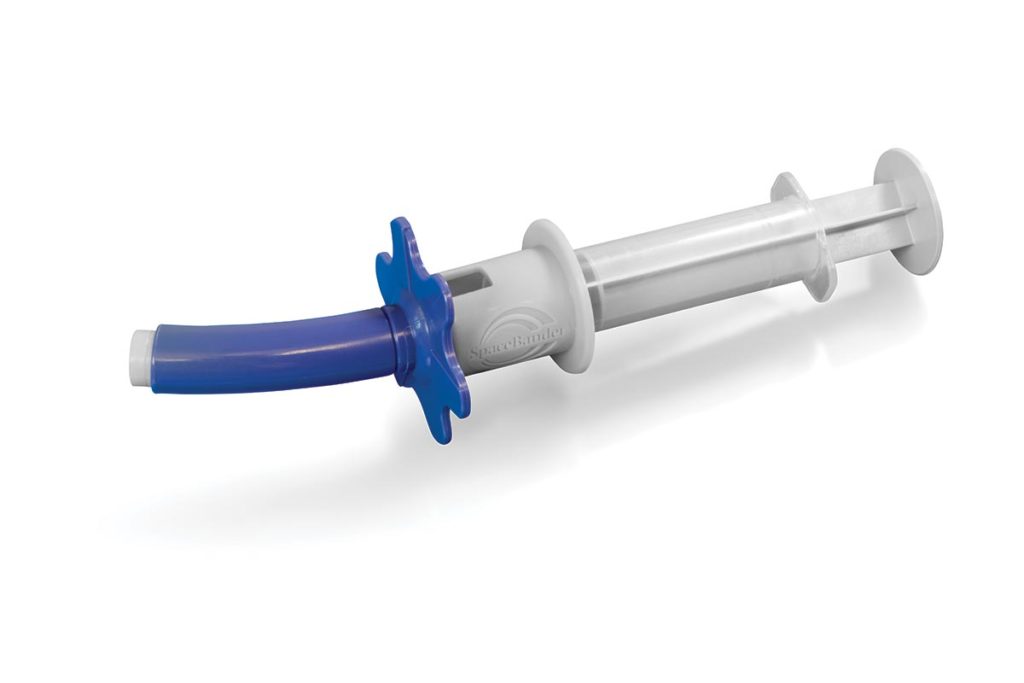
This device provides constant pressure to a syringe along with a separate metering device that allows a home user to administer drugs and medications. Multiple plastic injection molded parts are used in the molding process that include Noryl, Acetal, and Nylon materials. Taurus Engineering maintains a complete Device History Record (DHR) of all materials, components, labeling & packaging while managing all the components required for the device.

Single use surgical device intended to be utilized for the ligation and division of vessels, soft tissue, and lymphatics during minimally invasive and open procedures. The molding process includes a soft touch plastic injection overmolding with secondary pad printing. The first shot material is Nylon 66 with a VersaFlex overmold.